
Implications of Chronic Kidney Disease on Anaesthesia Management
Implications of Chronic Kidney Disease on Anaesthesia Management

Honorary Secretary
(2013-2015)
ISA – Nagpur City Branch
Introduction:
Chronic Kidney Disease (CKD) definition and nomenclature as given by Kidney Disease – Improving Global Outcome (KDIGO) includes abnormalities of kidney structure and function, present for more than three months, with implications for health. CKD is classified based on Cause, GFR category and Albuminuria category (CGA).
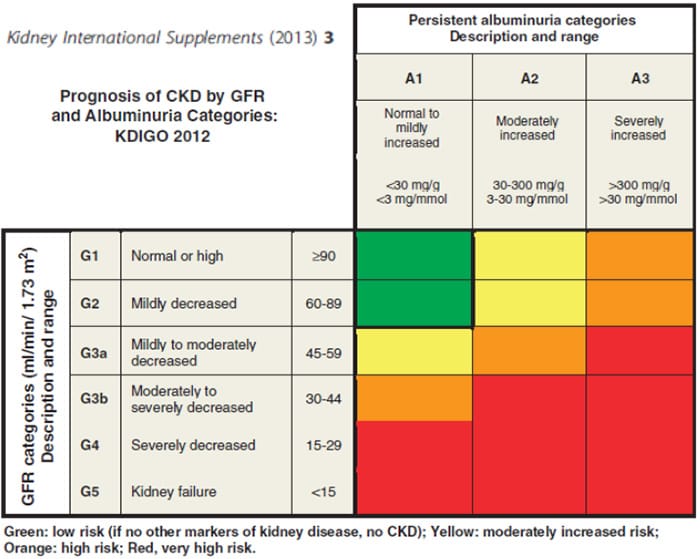
Apart from primary renal diseases, Diabetes Mellitus, Hypertension and
uncertain causes can lead to CKD. Patients of CKD are prone to serious
postoperative complications which include Acute Renal Failure and Cardiovascular
complications leading to increased morbidity and mortality.
Complications associated with CKD which have implications on safe conduct of anesthesia:
Cardiovascular System:
- Salt and water retention causing hypertension worsening and LVH
- Cardiomyopathy, congestive cardiac failure, subclinical pulmonary edema
- Accelerated atherosclerosis with stiffening of large capacitance
arteries - Myocardial fibrosis, conduction abnormalities
- Uremic pericarditis
- Autonomic Neuropathy causing sympathetic hyper-reactivity and
parasympathetic dysfunction - Anemia
- Calcification of valves
Coagulation system:
- Uremic thrombocytopathy
- Hypercoagulopathy
- Reduced fibrinolysis
- Vascular access thrombosis
Endocrine System:
- Secondary and Tertiary Hyperparathyroidism
- Vitamin D deficiency
- Diabetes Mellitus
Gastrointestinal System:
- Delayed gastric emptying
- Anorexia, vomiting, decreased protein intake, malnutrition
- Reduced Calcium absorption
Fluid and Electrolyte homeostasis:
- Hyperkalemia
- Volume overload / dehydration
Musculoskeletal System:
- Renal osteodystrophy
- Rhabdomyolysis after major surgery
Metabolism:
- Metabolic acidosis
- Abnormal Calcium & phosphate metabolism
- Muscle wasting, Negative nitrogen balance
Left ventricular hypertrophy could be secondary to pressure as well as volume
overload. Salt and water retention further adds to volume expansion. Myocardial
fibrosis and reduced compliance of LV causes diastolic dysfunction. Sudden
increase in preload and / or tachycardia can precipitate acute pulmonary edema.
Sympathetic hyper-reactivity can complicate further.
Adequate control of blood pressure is important and targets depend on
presence of proteinuria. If urine protein/creatinine ratio > 100 mg/mmol,
arterial blood pressure < 130/80 mm of Hg is beneficial. Blocking of
renin-angiotensin system using angiotensin converting enzyme inhibitors (ACEI)
or angiotensin receptor blockers (ARBs) has prognostic benefits apart from
controlling hypertension. Hence most of the CKD patients receive either ACEI or
ARBs or both. In the perioperative period, hypotension occurring in patients
receiving either or both these drugs may be refractory to treat. Myocardial
infarction, heart failure and stroke are leading causes of death in patients of
CKD.
Hypercoagulable states and reduced fibrinolysis increase the chances of deep
venous thrombosis. Antifibrinolytics like Tranexamic acid should be used
cautiously. Use of DVT pumps in prolonged surgeries could be beneficial. The
combination of uremic platelet dysfunction and residual effect of heparin in
post dialysis period mandates extra precaution while using neuraxial anesthesia.
Inability to excrete acids in CKD results in metabolic acidosis.
Administration of large volumes of saline leads to hyperchloremic acidotic
states. The deleterious effects of metabolic acidosis include depression of
myocardial contractility, decreased cardiac output and reduced renal blood flow.
Metabolic acidosis decreases albumin binding of drugs and increases percentage
of unbound drugs, especially local anesthetic agents. Albuminuria further
complicates the issue leading to high concentration of free drug and increased
chances of toxicity of drugs.
Patients with CKD can develop hyperkalemia if challenged with administration
of excess potassium or release from intracellular compartment (seen
post-fasciculations after succinyl choline administration). Acidemia, insulin
deficiency, hypertonicity and acute beta adrenergic blockade can precipitate
hyperkalemia.
Fluid retention in body leads to excess volume of distribution and altered
pharmacodynamics and pharmacokinetics of drugs leading to unpredictable effects
and duration of action. Kidneys contribute up to 18% of Cytochrome P450
activated drug metabolism. Also, non-renal clearance of many drugs is reduced in
kidney disease.
Anaesthesia Drugs:
Potent Inhalation agents:
Sevoflurane if used in higher concentrations (> 4MAC), can lead to renal
toxicity with formation of Compound A; but can be safely used in patients of CKD
in lower concentrations. Enflurane can cause vasopressin-resistant polyuria with
reduced urine concentrating ability and transient reductions in creatinine
clearance; so better avoided in patients of CKD. Desflurane and Isoflurane are
safer agents to be used in CKD as they are not associated with renal toxicity.
IV Induction agents:
Propofol pharmacokinetics are unaltered in renal failure. Thiopental has
increased volume of distribution and reduced plasma protein binding in renal
failure. The brain is exposed to higher free drug concentration. The rate of
administration of thiopental should be reduced.
Neuromuscular blocking agents:
The initial dose required to produce neuromuscular block is larger in patients with CKD than in normal subjects. But the dose required to maintain the block is reduced (except for atracurium & cis-atracurim). Pancuronium is longer acting drug mainly excreted through kidneys and also it has active metabolite – 3,hydroxypancuronium. The chances of post-operative residual curarization are higher if pancuronium is used in patients of CKD and better avoided. Though vecuronium undergoes predominantly biliary excretion, upto 30% drug may be excreted by kidneys. Renal failure results in reduced clearance, increased terminal half-life and prolonged duration of action. Rocuranium is also mainly excreted through bile with upto third being excreted through kidneys. Renal failure leads to similar effects on rocuronium metabolism as those of vecuronium; so both these drugs should be used cautiously in patients of CKD. Atracurium and Cis-atracurium do not depend on kidney or liver for metabolism. Their metabolism is pH and temperature dependent and occurs in plasma (Hoffman’s elimination). Metabolic product – Luadanosine is shown to have epileptogenic potential. Atracurium is less potent, has shorter duration of action, results in greater histamine release and has higher concentration of metabolite – Laudanosine than Cis-Atracurium.
Reversal Agents:
Neostigmine clearance is reduced and its half-life is prolonged in CKD. This
can lead to parasympathomimetic response which can cause bradycardia and AV
blocks – especially if used in combination with shorter acting atropine rather
than longer acting glycopyrrolate. Sugammadex is biologically inactive and
selectively encapsulates steroid based non-depolarising neuromuscular blocking
agents. Sugammadex is excreted unchanged in urine in individuals with normal
renal function. Its efficacy as reversal agent does not rely on renal excretion
of the cyclodextrin-relaxant complex.
Analgesic agents:
Acetaminophen in moderate dose used infrequently is safe in CKD. Prolonged
use can cause Analgesic nephropathy. In the postoperative period, acetaminophen
does not require dose adjustments.
Use of NSAIDs in perioperative period in CKD should be avoided as they can
exacerbate hypertension and precipitate edema, hyponatremia and hyperkalemia.
These drugs can cause acute decrease in GFR and may also lead to acute
interstitial nephritis as a part of their idiosyncratic reaction. Their drug
induced platelet inhibition along with uremic thrombasthenia, may cause
gastrointestinal bleeding.
Opioids do not cause any direct renal toxicity. But they have antidiuretic
activity and also can cause urinary retention. Minor metabolite of morphine, M6G
(morphine 6 glucuronoid) and approximately 5% of unchanged morphine in patients
of CKD, can cause delayed sedation & respiratory depression; therefore lower
dose should be used. Fentanyl and Alfentanyl do not have any active metabolite.
But their clearance is delayed in CKD and dose reduction is required.
Remifentanyl is not dependent on renal function as it is metabolized by
hydrolysis in plasma only. The use of meperidine in CKD is associated with
seizures, myoclonus and altered mental status because of reduced clearance of
its metabolite. Hemodialysis removes morphine and has no effect on fentanyl
while plasma concentration of remifentanyl is increased requiring reduction in
its infusion rate.
Almost 30% of Tramadol and active metabolite, O-demethyl tramadol are
excreted unchanged in urine. Uremia lowers seizure threshold and tramadol may be
epileptogenic in CKD. Hemodialysis removes tramadol.
Conclusion:
Chronic kidney disease is associated with systemic complications of almost
all organ systems of body. In the perioperative period, CKD patients should be
evaluated using system specific investigations so that optimization of system
function, risk stratification and risk factor modification can be done.
According to system functions, specific additional monitoring (apart from
routine) may be needed for smooth sailing in perioperative period. Perioperative
hemodialysis helps in optimizing patient condition. CKD may alter
pharmacokinetics and pharmacodynamics of anaesthetic drugs requiring dosage
modifications.
